Assistant Researcher Liu Jishan from the State Key Laboratory of Information Functional Materials, Shanghai Institute of Microsystems and Information Technology, Chinese Academy of Sciences and collaborators from the Northwest Pacific National Laboratory published the title Tuning the Electronic Structure of LaNiO3through Alloying with Strontium to Article of Enhance Oxygen Evolution Activitity. Liu Jishan is the first author of the paper, and Shanghai Microsystems is the first completion unit.
Oxygen evolution reaction (OER) plays an important role in the preparation and conversion of clean energy such as electrolyzed water and rechargeable metal-air batteries, and has received great attention. The kinetics of the oxygen evolution reaction is very slow and requires a high overpotential to proceed, which greatly limits the actual performance of the device. Currently, the most effective OER catalysts are IrO2 and RuO2. However, these precious metals and their oxides have been hindered by their wide application due to their high cost and shortage of resources. Therefore, the development of highly efficient and inexpensive OER catalysts based on 3d transition metals is the focus of current research. In this work, it was found that by incorporating 3D transition metal oxide lanthanum nickelate (LaNiO3) into the group II element strontium (Sr), its OER performance can be effectively improved. The results show that with the increase of Sr doping ratio, the catalytic activity of OER is significantly improved. At 1.6V, the OER current of 50% Sr-doped LaNiO3 is increased by 5 times compared with the undoped sample. At the same time, the overpotential required to produce an equal OER current is significantly reduced. Combining X-ray absorption spectroscopy (XAS), X-ray photoelectron spectroscopy (XPS) and DFT calculations, the introduction of Sr in LaNiO3 can shift the O 2p energy level to the Fermi energy level, thereby enhancing the Ni-O The hybridization intensity further reduces the charge transfer energy between Ni-O, and it is the change of its electronic structure that promotes the OER reaction process. The research in this paper confirms that the introduction of strontium doping in lanthanum nickelate is an effective way to improve its OER activity, and explains the reason for the increase in OER catalytic activity from the perspective of electronic structure, which provides a reference for the design of new and efficient catalysts in the future. .
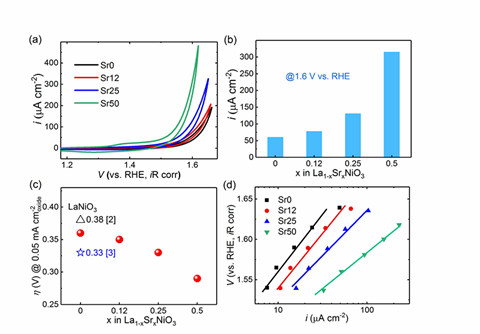
Comparison of OER characteristics of LNO under different Sr doping concentrations
Pp White Sheet,Pp Plastic Sheet,Grey Pp Sheet,Extruded Grey Pp Sheets
Heshan Liantuo Engineering Plastic Co.,Ltd , https://www.liantuoplastic.com
