Low-carbon olefins are important chemicals or intermediates, mainly derived from petrochemical processes such as naphtha cracking. Replacing petroleum-based basic chemicals with relatively low-cost and relatively abundant natural gas (the main component is CH4) is an important research and development direction in the current academic and industrial circles. CH4 is very stable. It is usually activated and oxidized by active oxygen species on the surface of the catalyst, but it is easy to overoxidize CH4 and its products to reduce the atom utilization rate. As an oxygen source, CO2 can also convert CH4 to lower olefins at higher temperatures, but catalyst deactivation is a key difficulty that has not yet been resolved.
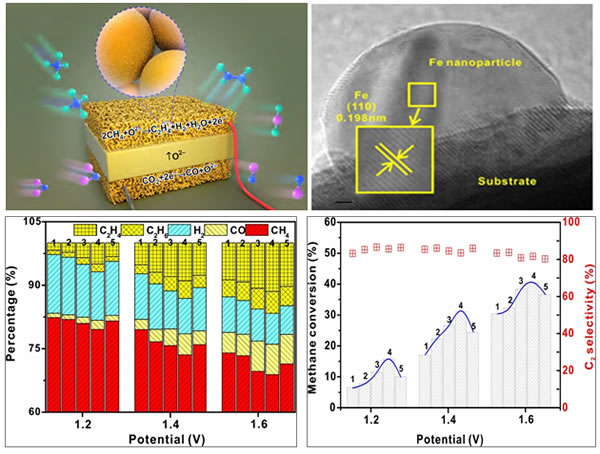
The research team of Xie Kui, Key Laboratory of Functional Nanostructure Design and Assembly, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, has developed under the support of the National Key R & D Program, the National Fund Major Research Program, the Joint Fund of the Clean Energy Innovation Institute of the Chinese Academy of Sciences, and the Fujian Provincial Hundred People Program A new way of catalyzing CH4 / CO2 to olefins by thermoelectric coupling is shown. As shown in the figure, CO2 is used as an oxygen source to activate and reduce to CO at the cathode, and oxygen ions are transported to the anode through the electrolyte to activate CH4 in the form of interfacial active oxygen and couple gas phase coupling To realize the conversion of CH4 to olefins. The coordinated modulation of the external field environment and interface catalysis avoids the excessive oxidation of CH4 and its products. This process not only realizes the conversion of CH4 to low-carbon olefins, but also realizes the reduction of CO2 and the utilization of high value, which is a new way with great potential of "two benefits, two benefits".
This work constructs a metal-oxide interface system by doping Sr2Fe1.5Mo0.5O6-δ (SFMO) and riveting and growing nano-iron on the surface to enhance the activation of CH4 and the stability of nano-metals and the resistance to carbon deposition. The length of the metal-oxide interface and the external electric field are coordinated to adjust the CH4 oxidation performance of the ceramic electrode. Under normal pressure, the C2 product concentration of 16.7% is achieved, the CH4 conversion rate can reach 41%, and there is no attenuation after 100 hours of operation. Related research results were published in Nature Communications.
The research group has previously carried out research work on a new interface system, electrolytic CO2 to CO and electrolytic CO2 coupled CH4 oxidation to syngas (Science Advances, 2018, 4, eaar 5100; Nature Communications, 2017, 8: 14785). Aiming at the active sites of small molecule activation and transformation, a long-range order of unsaturated coordination local structure is constructed to construct a porous single crystal system, and the mechanism of activation and transformation of specific small molecules such as coordination unsaturation and local electronic state is studied. (Advanced Mateirals, 2018, 1806552; Materials Horizons, 2018, 5,953-960; Nature Communications, 2017, 8, 2178).
CS20A Household Electric Screwdriver Set
SUZHOU CREATION SPACE INTELLIGENT TECHNOLOGY CO.,LTD , https://www.pkanglegrinder.com
